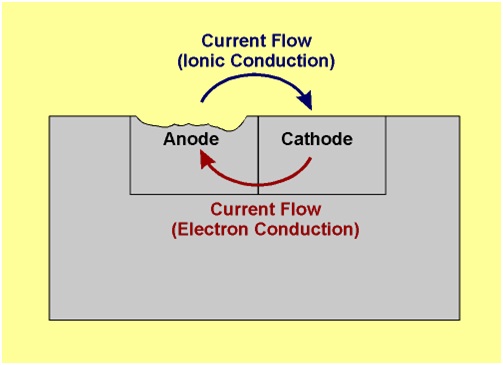
Zn ——– Zn 2+ + 2e- (Oxidation)
Thus it is evident that the corrosion occurs at the anode metal; while the cathodic part is protected from the attack.
Example:
- Steel screws in a brass marine hardware
- Lead-antimony solder around copper wise;
- a steel propeller shaft in bronze bearing
- Steel pipe connected to copper plumbing.


Leave a Comment