Electrochemical corrosion involves:
- The formation of anodic and cathodic areas or parts in contact with each other.
- Presence of a conducting medium.
- Corrosion of anodic areas only.
- Formation of corrosion product somewhere between anodic and cathodic areas. This involves flow of electron-current between the anodic and cathodic areas.
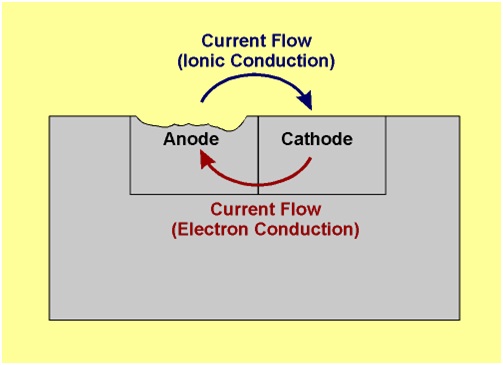
At anodic area oxidation reaction takes place (liberation of free electron), so anodic metal is destroyed by either dissolving or assuming combined state (such as oxide, etc.). Hence corrosion always occurs at anodic areas.
M (metal) → M n+ + n e-
Mn+ (metal ion) → Dissolves in solution
→ forms compounds such as oxide
At cathodic area, reduction reaction takes place (gain of electrons), usually cathode reactions do not affect the cathode, since most metals cannot be further reduced. So at cathodic part, dissolved constituents in the conducting medium accepts the electrons to form some ions like OH and O2-.
Cathodic reaction consumes electrons with either by
(a) evolution of hydrogen or
(b) absorption of oxygen, depending on the nature of the corrosive environment
The electrochemical corrosion is classified into the following two types:
(i) Galvanic (or Bimetallic) Corrosion
(ii) Differential aeration or concentration cell corrosion.
Galvanic Corrosion:
When two dissimilar metals (eg., zinc and copper) are electrically connected and exposed to an electrolyte, the metal higher in electrochemical series undergoes corrosion. In this process, the more active metal (with more negative electrode potential) acts as a anode while the less active metal (with less negative electrode potential) acts as cathode.
In the above example, zinc (higher in electrochemical series) forms the anode and is attacked and gets dissolved; whereas copper (lower in electrochemical series or more noble)acts as cathode.
Mechanism:
In acidic solution, the corrosion occurs by the hydrogen evolution process; while in neutral or slightly alkaline solution, oxygen absorption occurs.
The electron-current flows from the anode metal, zinc to the cathode metal, copper.
Zn ——– Zn 2+ + 2e- (Oxidation)
Thus it is evident that the corrosion occurs at the anode metal; while the cathodic part is protected from the attack.
Example:
- Steel screws in a brass marine hardware
- Lead-antimony solder around copper wise;
- a steel propeller shaft in bronze bearing
- Steel pipe connected to copper plumbing.


Leave a Comment