Applications:
- Protection as buried pipelines, underground cables from soil corrosion.
- Protection from marine corrosion of cables, ship hulls, piers etc.
- Insertion of magnesium sheets into the domestic water boilers to prevent the formation of rust.
- Calcium metal is employed to minimize engine corrosion.
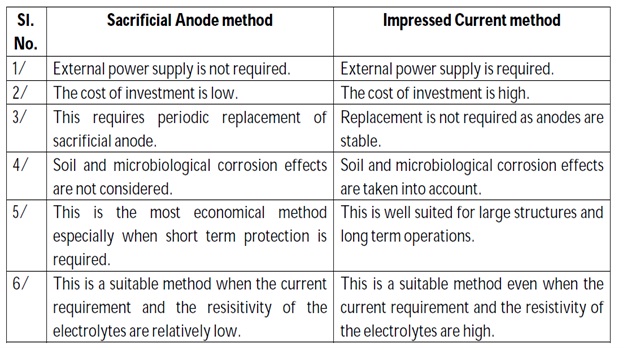
Advantages:
- Low installation and operating cost.
- Capacity to protect complex structures.
- Applied to wide range of severe corrodents.
Limitations:
- High starting current is required.
- Uncoated parts cannot be protected.
- Limited driving potential, hence, not applicable for large objects.
-
IMPRESSED CURRENT CATHODIC PROTECTION METHOD
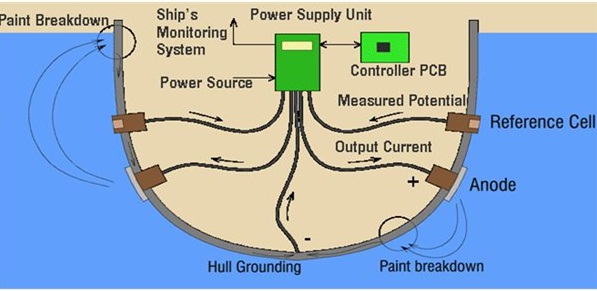
In this method, an impressed current is applied in opposite direction to nullify the corrosion current and convert the corroding metal from anode to cathode.
Usually the impressed current is derived from a direct current sources (like battery or rectifier on AC line) with an imsoluble, inert anode (like graphite, scrap iron, stainless steel, platinum or high silica iron).
A sufficient DC current is applied to an inert anode, buried in the soil (or immersed in the corroding medium) and connected to the metallic structure to be protected. The anode is, usually, a back fill, composed of coke breeze or gypsum, so as to increase the electrical contact with the surrounding soil.
Impressed current cathodic protection has been applied to open water box coolers, water tanks, buried oil or water pipes, condensers, transmission line towers, marine piers, laid up ships etc.
This kind of protection technique is particularly useful for large structures for long term operations.


Leave a Comment